All content credit due to our forum member hellouser for putting all the painstaking time and effort into attending the conference, and gathering all the audio and video content presented here.
9th World Congress for Hair Research 2015 – Miami, FL
Dr. Andy Goren’s presentation is below (and above). Several important questions are addressed in his presentation:
– Is it possible to know if minoxidil will work for you, before you try it?
– What are the factors that differentiate a responder to minoxidil from a non-responder?
– How does Minoxidil grow hair in the first place?
– Do higher concentrations of Minoxidil really work better?
– Can you grow hair on a bald spot, or hairless area of scalp?
We encourage you to follow along with the video, and discuss his findings in our forums:
Dr. Andy Goren – Minoxidil Response Testing in Females with Androgenetic Alopecia
Presentation: Minoxidil Response Testing in Females with Androgenetic Alopecia
Dr. Andy Goren – President, Chief Medical Officer of Applied Biology, Irvine CA
Speaker:
“We will have discussions for other talks after these presentations. I’m now happy to invite Andy Goren. Very great that we have him, and we switch a little bit back to the question of androgenetic alopecia and responsiveness to therapies. Andy Goren is president and CEO of Applied Biology and has a professorship at Rome.”
Dr. Andy Goren:
“Thank you. So, I’m going to talk about a subject that we sort of touched on, and I’ve heard over the years people have been talking about minoxidil response and drug response, in general, for growing hair. We started this project several years ago just trying to find some sort of marker that predicts hair growth.
This started as a project with plucked hair, and it’s turned out to be a little bit messy of the business with plucked hair. And that’s why I why mentioned this whole idea about normalization and the amount of hairs. People like me are losing hair, and don’t like a lot of plucking going on. And then, also it seems kind of cool to be in the laser. Doing something with laser. Might as well be in that, too. I have a lot of conflicts in general, but not in this particular case here.
I’m going to start with just: Topical minoxidil is the only FDA approved drug for female pattern in hair loss anywhere in the [inaudible]. Clinical response is about 24 weeks with the latest drug. 5% is the maximal approved concentration by FDA. Response is, at most, 30-40% anyways. When I say “response”, a lot of people come and say, “Well, works on a lot more women than that because it maintains hair growth,” but the response is actually defined as hair regrowths, not maintenance of hair. That’s the FDA label for this particular drug.
Because of the time that it takes, to actually get a response from this drug. And because it affects only; a very small amount of women would actually benefit (I think the 30% is probably on the higher side), it seems more for the male responders to that.
And, potentially, some other therapies that could be beneficial, including, as I mentioned, higher dose of minoxidil, which I’ll talk about during this talk. It would be great if there was some sort of response test ahead of time that we can just tell a woman, “Hey, this is going to work or not work.” We’re probably better at saying somebody is a nonresponder or a responder.
At least I don’t know, and I think most people don’t know what is the mechanism of actual minoxidil. There is some theories about it, but what we do know is minoxidil is actually not the active that causes the hair growths. It’s minoxidil sulfate. And minoxidil gets sulfate in the hair follicle on the outer root sheath, where the sulfotransferase SULT1A1 is expressed. With staining, you can see it’s there. And that’s probably, by the way, why a bald spot will not grow hair. You have to have some hair somewhere in order to get the sulfation working with minoxidil. This is the theory.
So we conducted several clinical studies actually for FDA in order to demonstrate the clinically [inaudible] validity of the sulfotransferase 1A1 as a marker for predicting response to minoxidil. We had 131 females. It was a prospective study with endpoints of plus hair counts and of, as I further said, primary endpoint. Secondary endpoint was global photography with expert assessment.
The subject used a 5% minoxidil topical foam, 24 weeks, once a day. And we utilized the assay that we recorded before and published several times which is colorimetric assay, where you immerse plucked hairs into the assay, and the sulfotransferase in the plucked hair converts minoxidil to minoxidil sulfate via PAP/PAPS system. And that you can read with a spectrophotometer and look at the optical density.
We used 8 plucked hair. Earlier, you mentioned we used 30 plucked hair. We used eight. The reason we used eight, is we found people who are losing hair were very reluctant to give us hair to begin with. Most of them think it won’t grow back. Probably does, but they don’t think it is.
The rationale, actually, for the eight plucked hairs: That was the minimal for the detection limit of 13.7. And where’d this 13.7 come from? So as far as I know, we’re the first one to actually have shown (and it’s not any rocket science) but: “What is the responder actually to minoxidil?” Because that’s another question: What does it mean you’re responding? Okay, you’re growing hair. How much hair are you growing?
According to the J&J studies, the last one they have done for minoxidil topical foam in women, you’ll see that one standard we chose, one standard deviation from the mean, for the placebo group was they don’t just report that any other way: this 13.7% increase in hair.
So what we have done is we have decided that if you are less than 13.7% increase in hair counts, then you’re a nonresponder. If you have more, then you are a responder. It’s a 0/1 type of thing. Primarily, okay. We have done 1 cm² photography with two of the patients and just the standard self with the global photography. We also investigated, rating was either one for hair regrowth or zero, which means they maintained hair loss. This is just a picture of the before and after.
So when we actually stratified the data – and this is an example of a scatterplot for that – if you see on – is it on this? Does this show on the screen? Wow, it’s very sophisticated. When you do this, we selected a biomarker here. This is with the sulfotransferase based on the optical density for the spectrophotometer. We just chose a number 0.4, which is any number other than one. But when we choose 0.4, you can see that most of the blue dots, which are the dots actually that relate to nonresponders, fall below the 0.4. What does that mean? That means we have a line here that shows we can probably find the nonresponders. And if we took that 0.4 and use some sophisticated Bayes’ Theorem, all sorts of mathematics, what we can find is that we can actually rule out, almost 98% of the time, subject will not nonresponse in minoxidil.
Now, we’re not very good at telling you if you’re going to respond. But it would make sense that if minoxidil sulfate is limited, if the sulfotransferase is a limited thing to produce minoxidil sulfate, then you don’t have enough of it or it doesn’t metabolize fast enough to minoxidil before it gets absorbed in your body. It will probably not work for you (minoxidil) in regrowing hair. So that’s what we’ve kind of show in this whole project.
The post Minoxidil: Why It Works for Some and Not For Others appeared first on HairLossTalk.
 Recently however huge news arrived of a partnership between
Recently however huge news arrived of a partnership between 



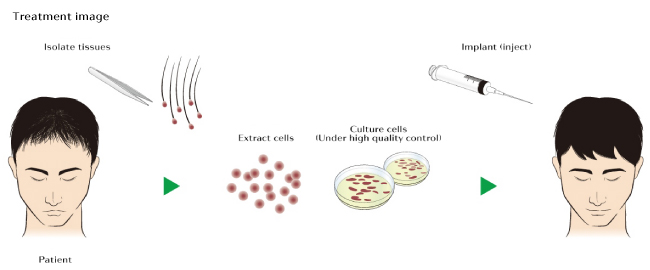
 So as you can see, its not just a bunch of electrodes taped to your scalp, but also includes growth factor infusions, which are currently being studied by several other teams on the “legit” front. The device itself seems to be an electrical stimulating device, intended to have an activating effect on stem cells, and likely circulation, which they hope contributes to the growth of new hair. Such devices have been attempted in the past (without the stem cell claim, and not in combination with growth factor infusions) and shown to be ineffective in the treatment of hair loss. So people are keeping a cautious eye on this treatment, and waiting for reproducible study data before raising hopes.
So as you can see, its not just a bunch of electrodes taped to your scalp, but also includes growth factor infusions, which are currently being studied by several other teams on the “legit” front. The device itself seems to be an electrical stimulating device, intended to have an activating effect on stem cells, and likely circulation, which they hope contributes to the growth of new hair. Such devices have been attempted in the past (without the stem cell claim, and not in combination with growth factor infusions) and shown to be ineffective in the treatment of hair loss. So people are keeping a cautious eye on this treatment, and waiting for reproducible study data before raising hopes.